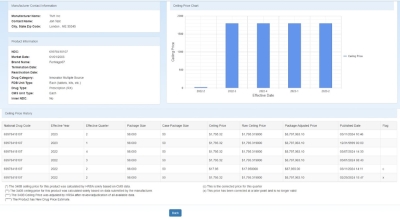
NDC Product Details
Upon clicking an ![]() NDC on the
NDC on the ![]() Ceiling Price page, the Product Details page is displayed.
Ceiling Price page, the Product Details page is displayed.
-
The left side of the page shows the Manufacturer Contact Information and Product Information.
-
The Manufacturer Contact Information section shows the manufacturer name, contact name and email address, and the manufacturer's city, state, and ZIP code.
-
The Product Information section shows the National Drug Code (NDC), market date, brand name, termination date (if applicable), drug category, FDB unit type, drug type, and
 CMS unit type.
CMS unit type.
-
-
The right side of the page contains the Ceiling Price Chart showing the ceiling price by effective year and quarter. The associated information is listed in grid format below the chart, including NDC, effective date ceiling price (AMP –
 URA), package adjusted price (ceiling price multiplied by the drug's package size and case package size).
URA), package adjusted price (ceiling price multiplied by the drug's package size and case package size).-
The asterisks (****) in the Flag column denote that the product is a New Drug Price Estimate
-
-
Hover over a bar in the ceiling price chart to view the associated price.
-
The following Flags denote that there has been a price correction: (c) – This is the corrected price for this quarter. (x) – This price has been corrected at a later point and is no longer valid.
-
Click the Back button to return to the Ceiling Price page.